Abstract
Introduction
CLL remains an incurable disease and represents a significant health problem in the western world. Increasing evidence highlights that the impact of marrow stromal cells is a key component influencing CLL B-cell survival. We have utilized an in vitro bone marrow stromal cell (BMSC) model system and found unique alterations in CLL B-cells with BMSC co-culture that point to previously unidentified biologic changes in the CLL B-cells that may influence CLL B-cell signaling and drug resistance.
Methods
Purified primary CLL B-cells (n= 39) from previously untreated CLL patients were cultured alone or co-cultured with primary BMSCs from either normal individuals (n=26) or CLL patients (n=17) at a 50:1 ratio in AIMV medium. After 48 hours, separated CLL B-cells or BMSCs were examined by immunoprecipitation/Western blot analyses and where needed real time PCR was done to assess the presence of intracellular proteins. In separate experiments to assess CLL B-cell killing, purified CLL B-cells were treated with TP-0903, fludarabine, chlorambucil and ibrutinib as single agents with or without BMSC co-culture.
Results
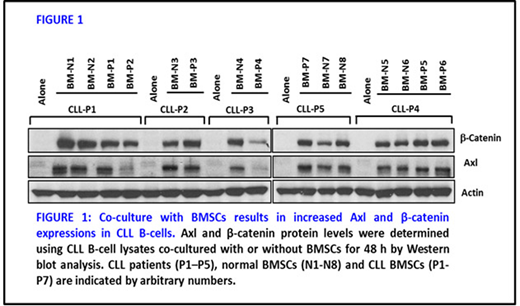
We observed significant increases in expression of Axl for both mRNA and protein levels in CLL B-cells co-cultured with BMSCs compared to CLL B-cells cultured alone. We also detected significantly increased expression of β-catenin at the protein level in CLL B-cells co-cultured with BMSC. But, we did not see any significant change in β-catenin or Axl protein expression in BMSCs co-cultured with CLL B-cells. Co-culturing of CLL B-cells with BMSCs using transwells confirmed that the upregulation of both Axl and β-catenin is dependent on the direct contact of CLL B-cells with BMSCs. The CLL B-cells from co-culture also had upregulation in phosphorylated (P)-ERK-1/2 but no change in P-AKT(Ser473). High nuclear β-catenin and P-ERK-1/2 levels were also detected in co-cultured CLL B-cells. ERK associates with and inactivates GSK-3β resulting in the up-regulation of β-catenin. We next checked for P-GSK-3β (Ser9) in co-cultured CLL B-cells. Upregulation in P-GSK-3β (Ser9) detected in co-cultured CLL B-cells suggests inactivation of GSK-3β and increasing β-catenin accumulation in co-cultured CLL B-cells. Moreover, inhibition of P-ERK-1/2 with inhibitor PD98059 in CLL B-cells cultured with BMSCs inhibited β-catenin as well as Axl expression levels. We also determined the phosphorylation status of Axl in CLL B-cells in co-culture with BMSC but found no change either at Y702 (Axl kinase domain) or total tyrosine phosphorylation levels for Axl in CLL B-cells. Thus, we assume that the role of Axl in co-cultured leukemic B-cells is independent of its kinase activity. Next we determined the effect of the highly specific Axl inhibitor TP-0903 on CLL B-cell status of Axl and b-catenin while in BMSC co-culture. Interestingly, both Axl and β-catenin protein expression levels were found to be further upregulated in CLL B-cells exposed to sub-lethal doses of TP-0903 in co-culture with BMSC. Treatment with chemotherapeutic or targeted therapy drugs, (i.e. fludarabine, chlorambucil or ibrutinib) also led to increase in expression levels of both β-catenin and Axl CLL B-cells co-cultured with BMSC. Of interest CLL B-cells were less sensitive to the chemotherapy drugs in presence of BMSCs, suggesting a role for both Axl and β-catenin in stromal mediated CLL B-cell drug resistance to these agents. This was not true for the Axl inhibitor as TP-0903 was able to induce robust cell death by targeting P-Axl and overcome BMSC mediated protection even in the presence of increased Axl and b-catenin. We also found that TP-0903 decreased P-Axl as well as the Axl downstream mediator, P-Akt(S473) and reduced Mcl-1 expression in CLL B-cells in BMSC co-culture.
Conclusions
Here we show that marrow stromal cell mediated increased expression in both β-catenin and Axl in CLL B-cells is associated with leukemic B cell survival and drug resistance. The mechanism for this may in part be via activated ERK levels that also occur when CLL B-cells contact BMSC. The BMSC resistance appears to be more profound for chemotherapeutic agents since Axl inhibitor can still induce CLL B-cell killing with BMSC co-culture. These studies suggest that a further understanding of the roles of Axl and β-catenin in the resistance status of CLL B-cells mediated by contact with BMSC are warranted.
Warner:Tolero Pharmaceuticals: Employment. Bearss:Tolero Pharmaceuticals, Inc: Employment. Kay:Morpho-sys: Membership on an entity's Board of Directors or advisory committees; Cytomx Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Infinity Pharm: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Acerta: Research Funding; Tolero Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios Pharm: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal